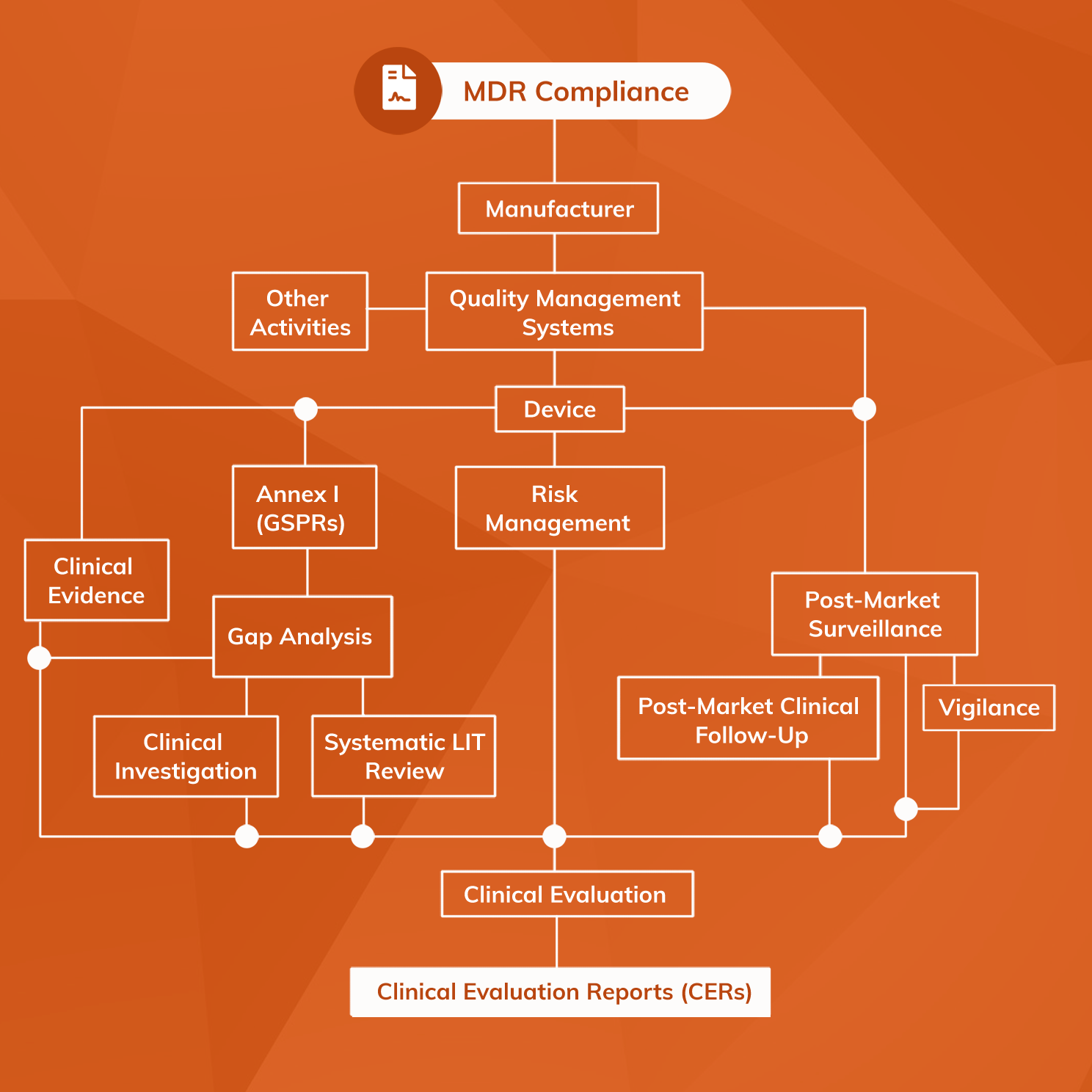
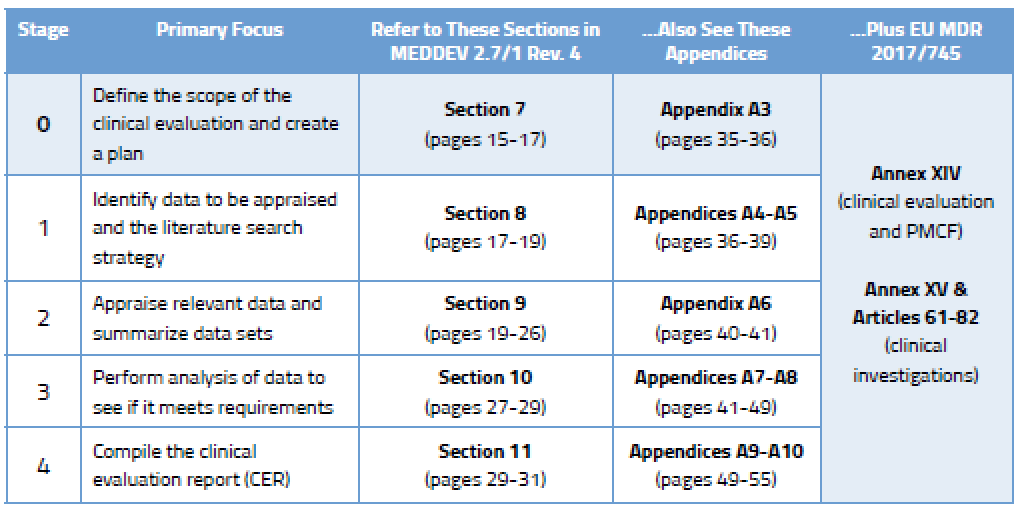
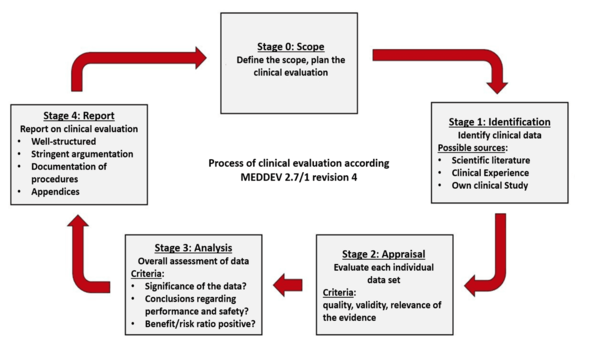
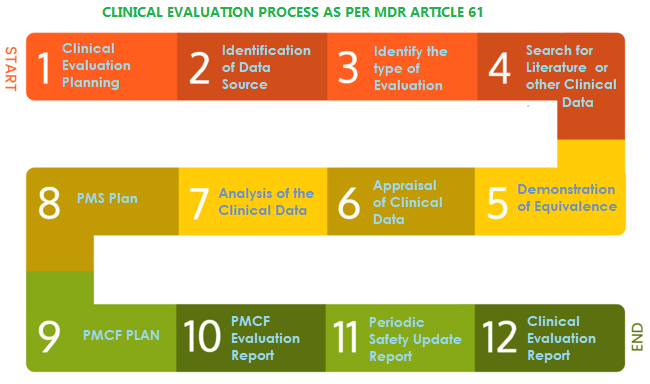
How to perform a clinical evaluation of medical devices – Part 3 – Suggested Table of Contents for the Clinical Evaluation Report – CER – Medical Device Expert News

How to perform a clinical evaluation of medical devices – Part 1 – Overview and sample of activities – Medical Device Expert News













.png.aspx)
%20The%20Ultimate%20Guide%20to%20Clinical%20Evaluation%20of%20a%20Medical%20Device.png?width=300&name=(cover)%20The%20Ultimate%20Guide%20to%20Clinical%20Evaluation%20of%20a%20Medical%20Device.png)