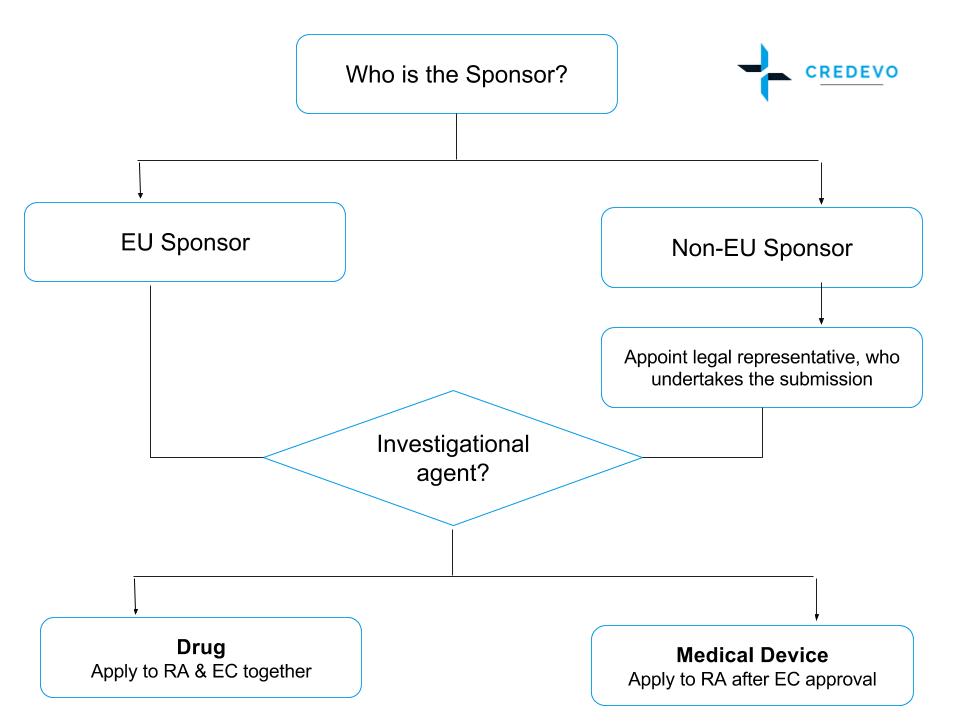
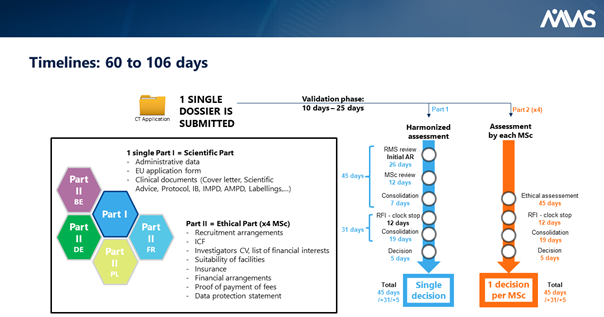
Applying to the Medicines and Healthcare Products Regulatory Agency for a Dentists, Doctors Exemption Certificate (DDX) or a Cl
Annex 1: Clinical trial Application Form The questions in this form for the request for authorisation from the Competent Authori