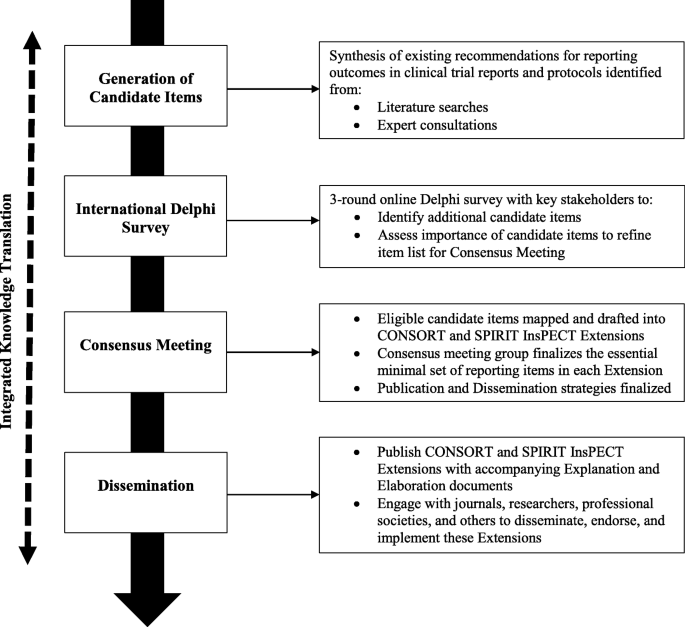
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
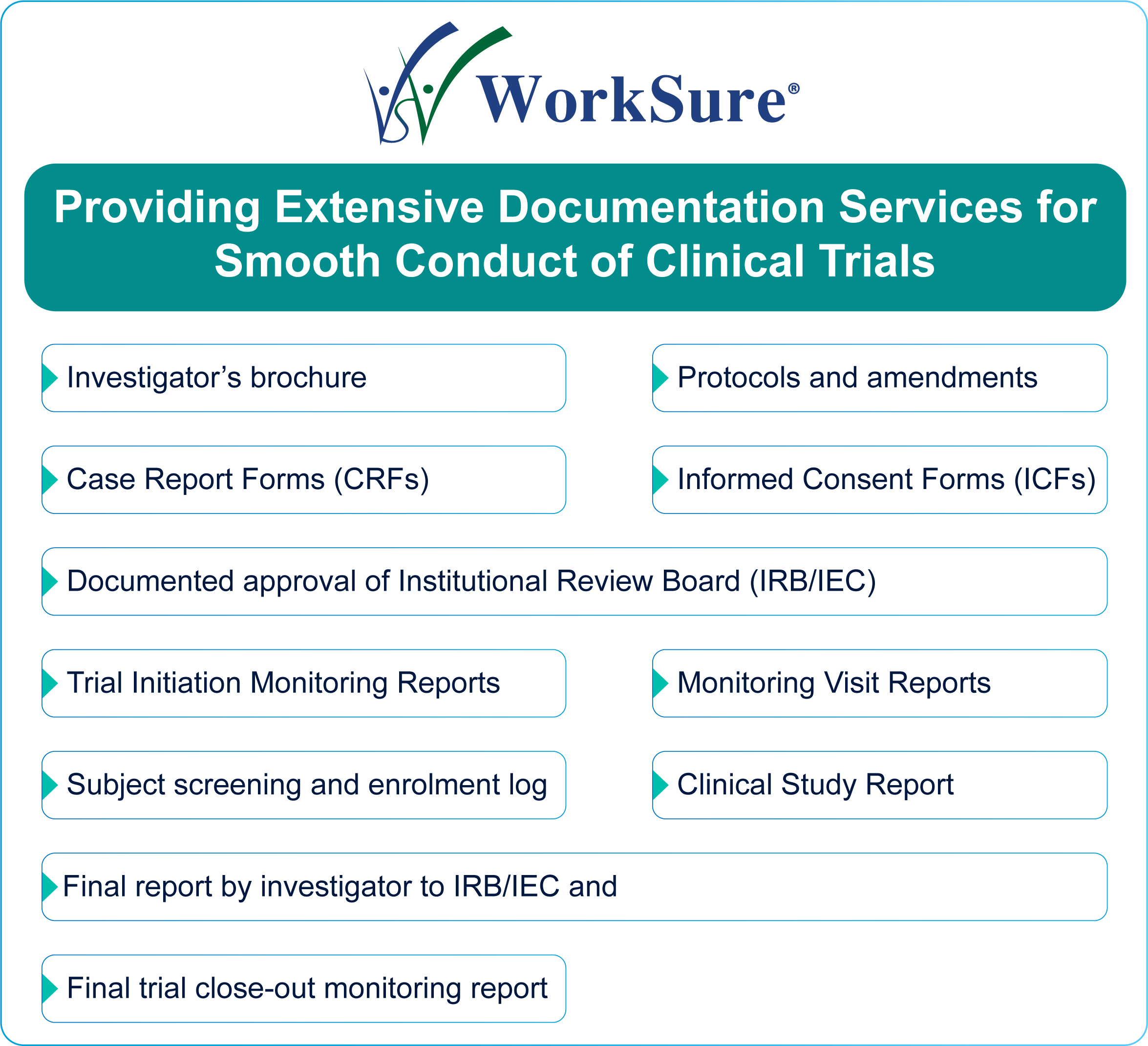
Role of Extensive Documentation in Smooth Conduct of Clinical Trials | WorkSure™ MedPharma Consultancy India Pvt. Ltd.















