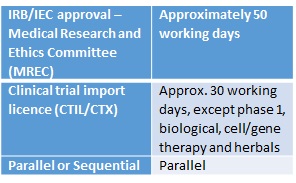
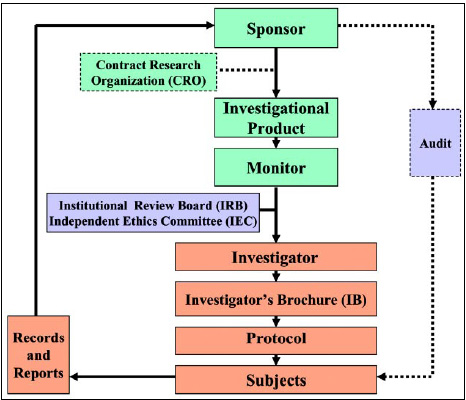
Current clinical trial approval process in India. Abbreviations: BM,... | Download Scientific Diagram
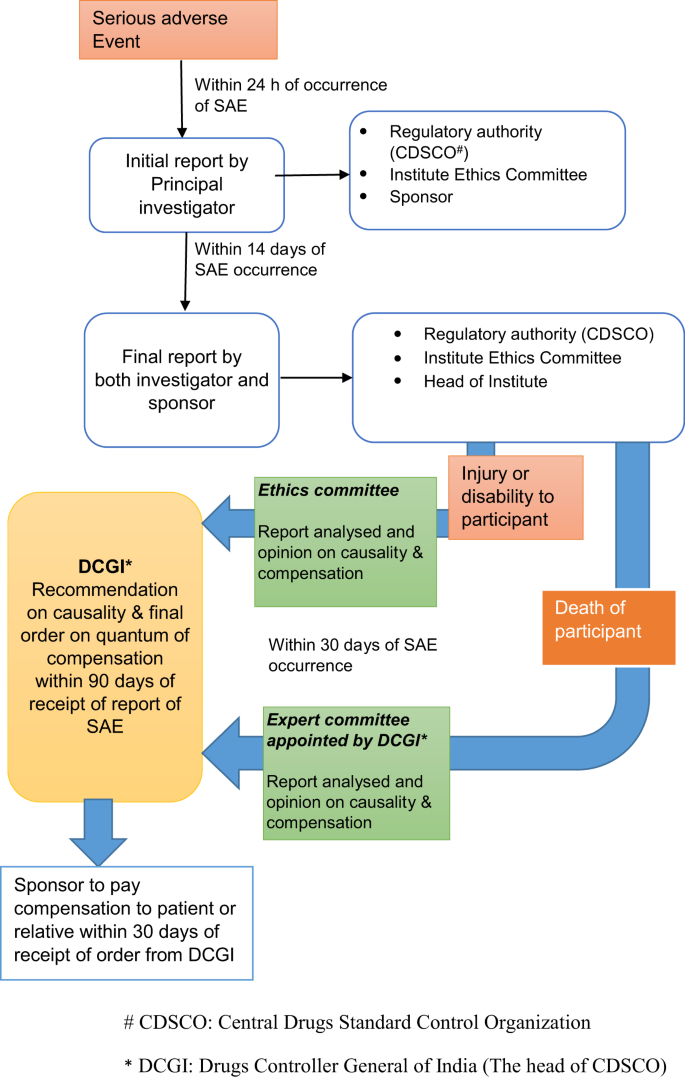
Issues, challenges, and the way forward in conducting clinical trials among neonates: investigators' perspective | Journal of Perinatology