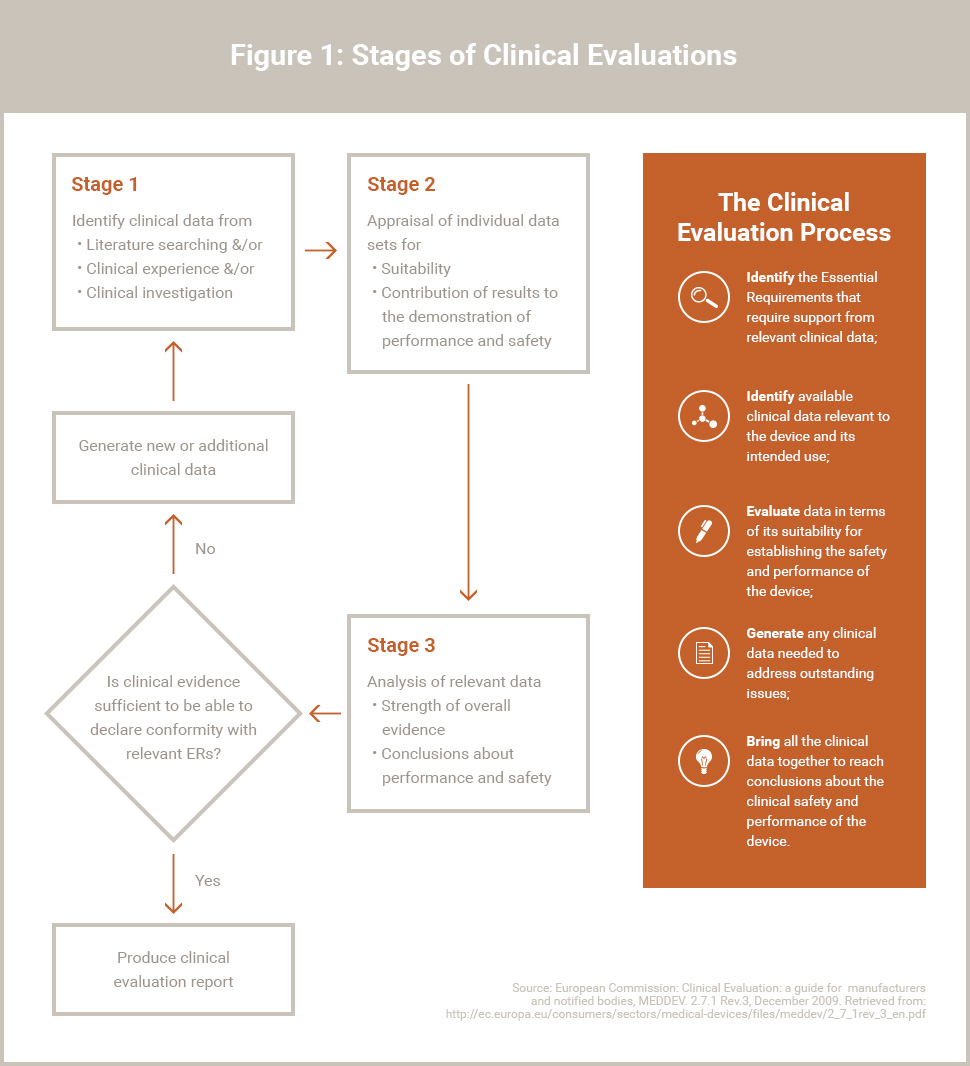
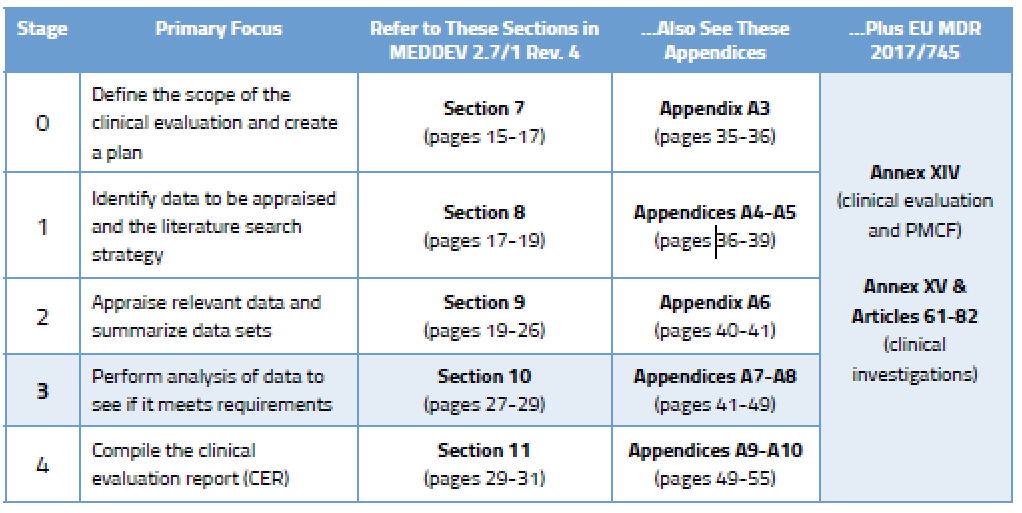
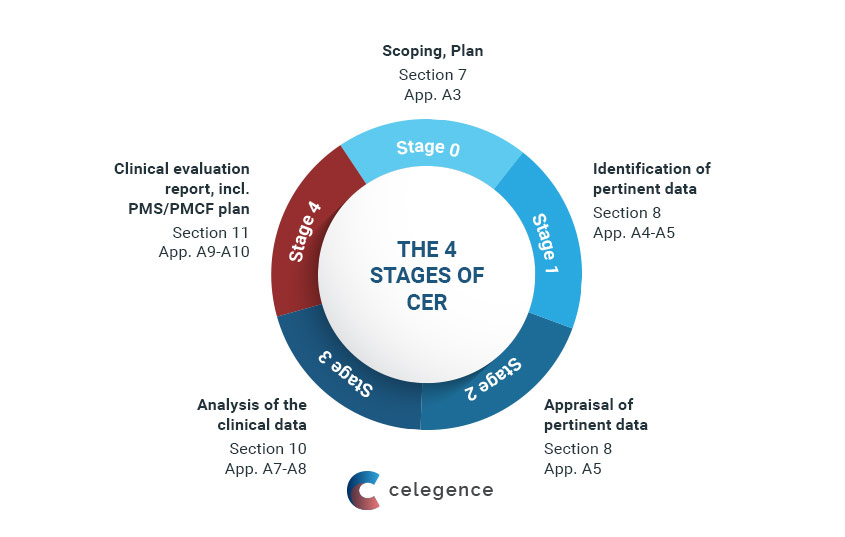
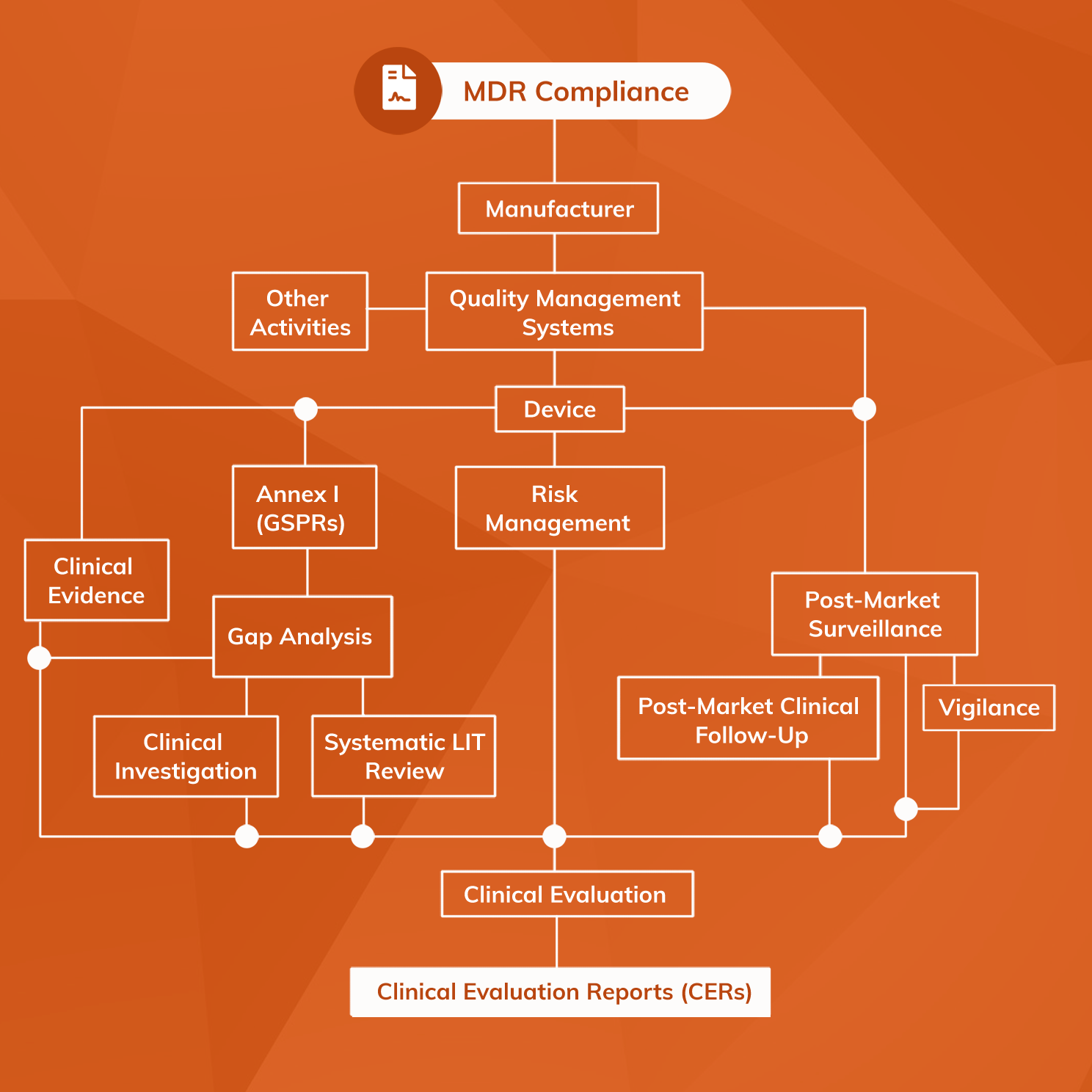
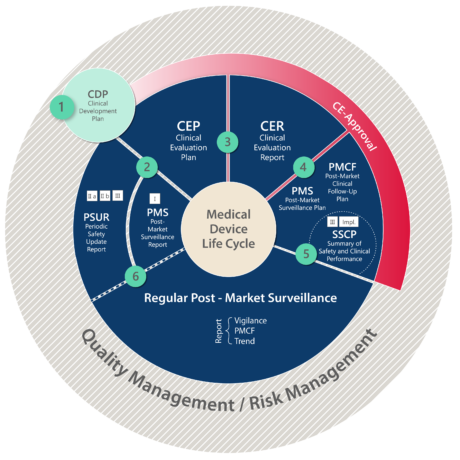
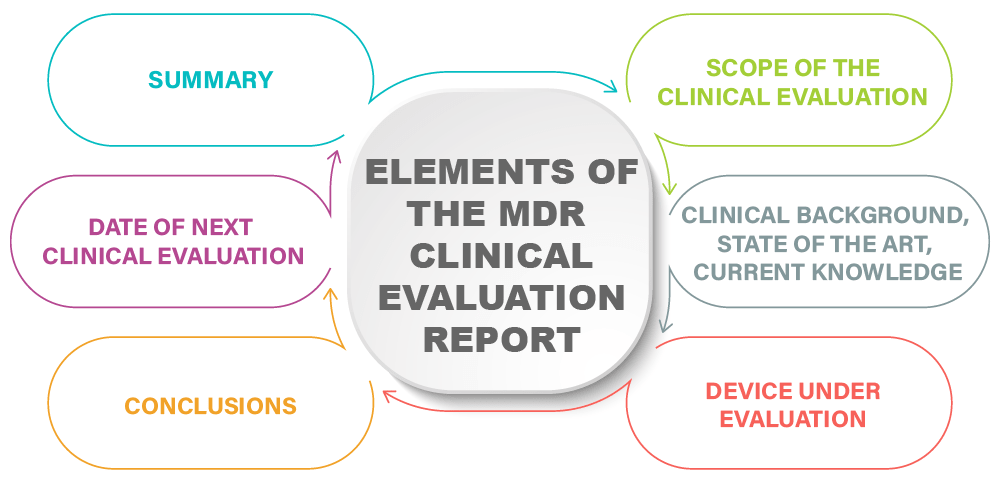
Clinical Evaluation Report for Medical Devices-Role of Medical Writer – Turacoz Healthcare Solutions

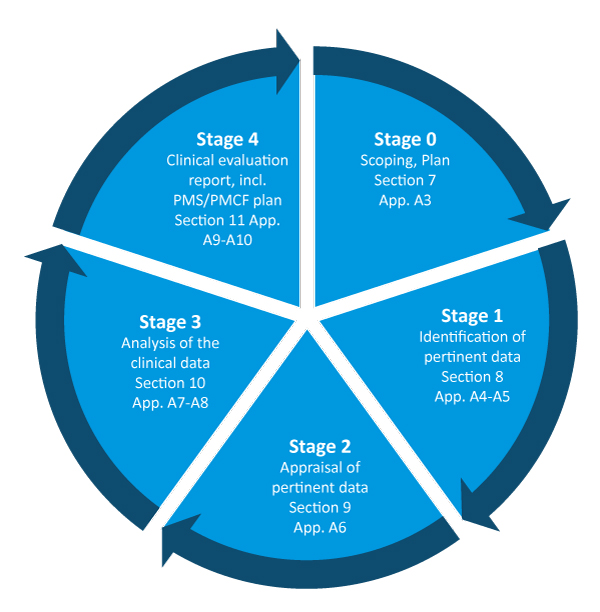
Clinical Evaluation of a Medical Device: Creating a Process and Establishing Equivalency (Part 1 of 4)
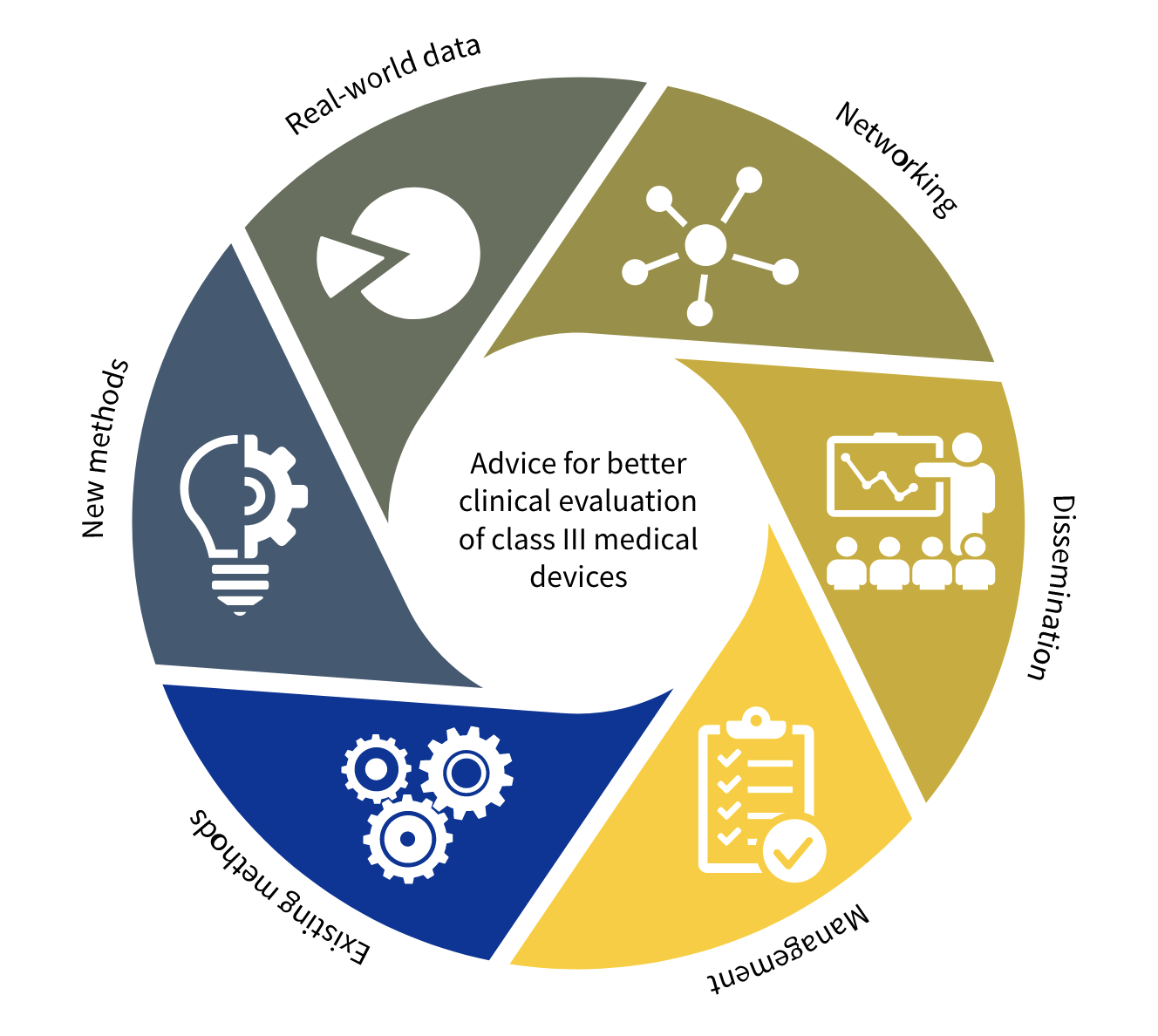
White Paper Clinical Evaluation of Medical Devices: The Increasing Responsibilities of Clinical Evaluators
White Paper Clinical Evaluation of Medical Devices: The Increasing Responsibilities of Clinical Evaluators





![PDF] Clinical evaluation of medical devices: main constraints and specificities. | Semantic Scholar PDF] Clinical evaluation of medical devices: main constraints and specificities. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6e19c2250d1ca153b8d8e82bb8a7354426a10932/4-TableII-1.png)